New 2025 AHA/ACC guidelines recommend
Renal Denervation to lower blood pressure.
It’s not another pill, It’s another way.
The 2025 AHA/ACC Hypertension guideline recognize Renal Denervation (RDN) as an adjunct therapy for appropriately selected patients with uncontrolled or resistant hypertension.
When RDN should be considered:
-
Patients with stage 2 hypertension (office SBP ≥ 140mmHg and DBP≥ 90 mmHg) who are unable to take antihypertensive medications at the optimal dosages or additional medication
-
Patients with stage 2 hypertension in whom BP is not at goal despite taking ≥4 antihypertensive medications at optimal dosages (ACEi/ARB + CCB + thiazide-type diuretics, and MRA)
Global hypertension guidelines now recommend RDN as an adjunct treatment for hypertension.
| Guidelines | AHA 20251 | ESC 20242 | EHS 20243 |
| Level of Evidence | 2b | llb | ll |
| Position on RDN | Adjunct option for Resistant & Uncontrolled Hypertension | Adjunct option for Resistant & Uncontrolled Hypertension | Adjunct option for Resistant & Uncontrolled Hypertension |
| Patient selection |
|
|
|
| Multidisciplinary team/Shared decision making | |
|
|
| Intolerant and/or non- adherent to drugs |

"These results show that ultrasound renal denervation can deliver meaningful and sustained blood pressure reductions even in real-world settings—without increasing medication burden. It’s a powerful validation of the therapy’s potential and a promising step toward broader adoption in the management of hypertension.”
— Dr. Michael J. Bloch
University of Nevada School of Medicine
& Renown Institute of Heart and Vascular Health, NV
Stay ahead in hypertension care.
Gain exclusive insights into the Paradise Ultrasound Renal Denervation procedure and cutting-edge strategies for hypertension management. Dr. Bloch breaks down the science, shares clinical experience, and offers practical guidance you won’t find anywhere else.
Elevate your understanding of this transformative therapy.
Experience a new path to lowering blood pressure.
The ParadiseTM Ultrasound Rendal Denervation Procedure
Drive to blood pressure goal.
Paradise Ultrasound RDN procedure treats hypertension by calming the overactive renal nerves. When medications and lifestyle changes are not enough, it delivers safe and sustained reductions proven across multiple sham-controlled trials.4-7
The Paradise uRDN procedure works by delivering ultrasound energy to renal arteries to disrupt overactive sympathetic nerves, reducing nerve signaling that contributes to high blood pressure.
It is a short procedure and is not an implant. Patients go back to referring physicians for follow-up.

Learnings from a real conversation with Dr. Brown: her insights on patient selection criteria for renal denervation.
Dr. Angela Brown breaks down how she identifies the right candidates for Renal Denervation.
Take control—check if the Paradise uRDN procedure is right for your patients.
- Patients who have elevated blood pressure despite 3 or more medications.
- Patients who are on multiple medications and have documented non-adherence/intolerance.
- Patients who may have a history of hypertensive crisis and/or at high risk of cardiovascular events.
*See Important Safety Information at the bottom of this page. Refer to the IFU for more information prior to considering the Paradise uRDN procedure.
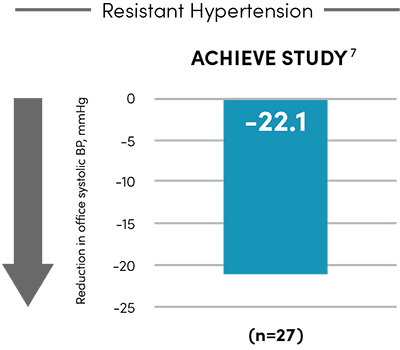
A proven approach to managing hypertension.
-10.4
mmHg
Office systolic blood pressure at 2 months4
(n=293)
RADIANCE Pooled Results
-17.7
mmHg
Office blood pressure at 3 years8
(n=51)
RADIANCE SOLO (Off-MED study)
Trust in Proven Safety
A one-time, minimally invasive, outpatient procedure delivering meaningful blood pressure reductions4-7.
Proven safe with no impact to kidney function8, and without side effects of drugs.
Safe treatment proven procedural protection
ZERO
major adverse events in the pivotal trial7
NO
impact on renal function at 2 months4
ZERO
clinically significant renal arterial stenosis at 6 months4
Deliver lasting outcomes.
Transforming uncontrolled resistant hypertension with 24-hour reduction4-7, a meaningful blood pressure drop at 6 months, sustainable improvement for years to come.8,9
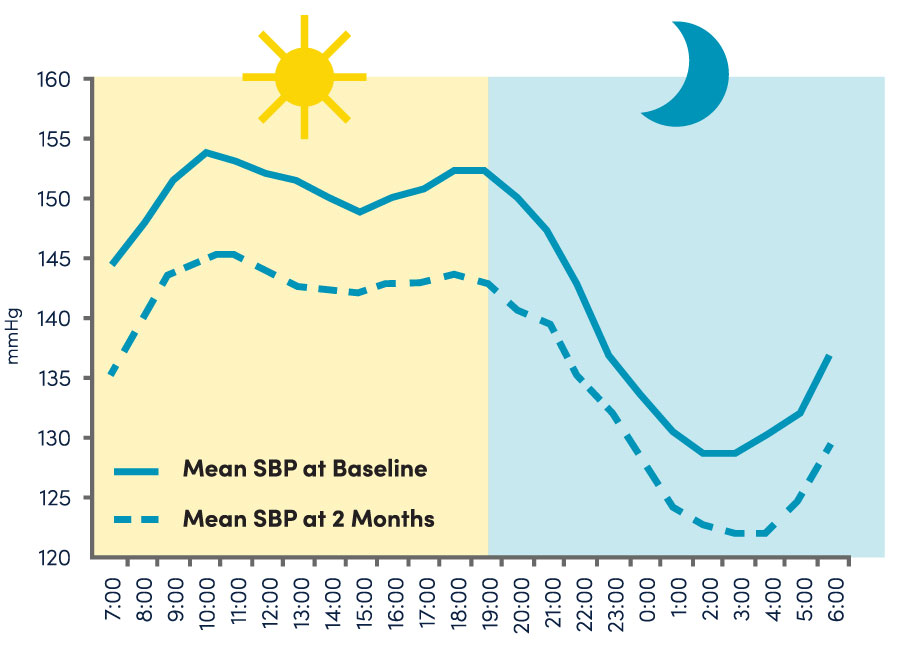
Paradise procedure led to a sustained reduction in office systolic blood pressure through 8 years.9
References:
1. Jones D, et all, Hypertension, https://doi.org/10.1161/HYP.0000000000000249 2. European Heart Journal, ehae178, https://doi.org/10.1093/eurheartj/ehae178 3. Mancia et al. J Hypertension 41(12):p 1874-2071, December 2023 4. Kirtane A. et al. JAMA Cardiol. 2023;8(5):464-473 5. Azizi M. et al. Lancet. 2018 Jun ;391(10137):2335-2345. 6. Azizi M. et al. Lancet. 2021 Jun 26;397(10293):2476-2486. 7. Azizi M. JAMA. 2023;329(8):651-661. 8. Radar et al. EuroIntervention 2022;18-online 9. Daemen et al. ACHIEVE study. J Hypertens. 2019 Sep;37(9):1906-1912
Important Safety Information Rx Only. Brief Summary – Prior to use, please reference the Instructions for Use.
Indications for Use
The Paradise Ultrasound Renal Denervation System (Paradise System) is indicated to reduce blood pressure as an adjunctive treatment in hypertension patients in whom lifestyle modifications and antihypertensive medications do not adequately control blood pressure.
Contraindications
The Paradise Catheter is contraindicated in any of the following:
• Renal arteries diameter <3 mm and >8 mm • Renal artery Fibromuscular disease (FMD) • Stented renal artery • Renal artery aneurysm • Renal artery diameter stenosis >30% • Pregnancy • Presence of abnormal kidney (or secreting adrenal) tumors • Iliac/femoral artery stenosis precluding insertion of the catheter.
Warnings
• Failure to use the recommended balloon size may result in renal artery stenosis, dissection, perforation, aneurysm, significant vasospasm requiring intervention, ablation of unintended
tissues or structures, and/or no ablation of target tissue achieved. • Energy emission in an unintended location may result in unintended tissue damage. • Do not move the Paradise
Catheter during sonication. • Do not sonicate in renal artery at locations with visible plaque. • Do not deliver sonications in an overlapping arterial target zone.
Potential risks of renal denervation procedure/response to treatment
Ablation or thermal injury to vessel, adjacent tissue or other structures, Acute kidney injury, Angina, Anxiety, Arrhythmia, Atrial tachycardia, Bradycardia, Gastrointestinal complications (diarrhea, nausea, vomiting), Hypotension/ Dizziness and/or Headaches, Hypertension, Hyperhidrosis, Pain (transient abdominal, lower back), Renal failure or renal insufficiency, Renal artery aneurysm or pseudoaneurysm, Renal infarction, Renal artery dissection, or perforation, Renal artery stenosis, Vasospasm, Vasovagal response, Stroke or transient ischemic event
Potential risks of arterial catheterization procedure
Allergic reaction to contrast, Arterio-enteric fistula, Arterio-venous fistula, Bleeding, Cardiopulmonary arrest, Complications related to pain and anti-anxiety medications, Death, Deep vein thrombosis, Edema, Embolism (pulmonary, renal, peripheral vasculature, plaque), Hematuria, Infection, Myocardial infarction, Pain, Vascular access site complications (pseudoaneurysm, pain, swelling, hematoma)